Bioimaging center Lille (BICeL)
Une expertise en microscopie photonique et histologie, microscopie électronique, cytométrie et imagerie en flux pour les études fonctionnelles dynamiques, de la molécule jusqu'au petit animal.
Bioimaging center Lille (BICeL)
Une expertise en microscopie photonique et histologie, microscopie électronique, cytométrie et imagerie en flux pour les études fonctionnelles dynamiques, de la molécule jusqu'au petit animal.
Bioimaging center Lille (BICeL)
Le BiCeL est une plateforme académique spécialisée en microscopie photonique et histologie, microscopie électronique, cytométrie et imagerie en flux. Membre de l’unité PLBS (UAR2014 US41), qui rassemble les plateformes lilloises en biologie et santé, il présente une structuration multisites et multi-technologies, regroupant 20 personnes sur le campus santé, la cité scientifique et l’Institut Pasteur de Lille.
Le BICeL soutient la recherche locale, nationale et internationale, offrant des technologies avancées au service des problématiques en biologie et santé. Il rend accessible un parc de 38 équipements, notamment d’imagerie multimodale, pour l’analyse de phénomènes biologiques complexes en temps réel. Ces outils sont utilisés sur un large panel d’échantillons, englobant microparticules et nanoparticules, bactéries, mollusques, plantes, insectes, poissons et mammifères, dans des conditions de sécurité biologique adéquates. L’expertise du BICeL permet un accompagnement individualisé, du conseil à la prestation de service complète. Le positionnement de la plateforme au sein de l’unité PLBS favorise par ailleurs le développement de projets aux interfaces technologiques.
Expertises et services
- Imagerie photonique, histologie, imagerie électronique et cytométrie en flux,
- Imagerie intravitale et microscopie confocale multiphoton en animalerie A1 et A2,
- Conseil pour la préparation d’échantillons et la sélection de systèmes appropriés,
- Aide à l’acquisition de données, à leur analyse et interprétation,
- Formation théorique et pratique aux techniques spécifiques utilisées sur la plateforme,
- Prise en charge partielle ou complète de projets : préparation d’échantillons, acquisition, analyse et interprétation des données,
- Mise à disposition d’équipements, cytomètres analyseurs, cytomètres trieurs et spinning disk à haute résolution sous environnement contrôlé de niveau de biosécurité 2,
- Mise à disposition d’outils de transfert et de gestion des données générées.
Moyens et équipements
Microscopie électronique :
- 3 ultramicrotomes dont 2 équipés pour les cryo-coupes,
- 2 appareils de cryo-substitution,
- MET 80-120 kV,
- MET 200 kV LaB6 avec tomographie, cryo, STEM, EDX, EELS, GIF et caméra Ultrascan 4K,
- MEB avec cryo-station et serial block face (SBF) 3View.
Microscopie photonique :
- Appareils de préparation de coupe et coloration en histologie,
- Scanners de lames et imageur en plaque,
- Microscopes plein champ et vidéomicroscopes,
- Microscopes confocaux LSM ou spinning disk, dont plusieurs AiryScan, X-Light, et un Live-SR équipé pour le FRAP, FRET et TIRF,
- Microscope de super résolution STED,
- Microscope à feuille de lumière,
- Microscope multiphotons/SHG,
- Système d’imagerie du petit animal pour rongeur.
Cytométrie et imagerie en flux :
- 3 analyseurs LSR Fortessa, LSR Fortessa X-20 et CytoFLEX LX,
- Analyseur acoustique Life Technologies Attune NxT,
- Analyseur spectral SP6800,
- Trieur conventionnel SH800,
- 2 trieurs spectraux Aurora CS.
Parc informatique :
- 12 stations de travail off-line,
- Solutions de stockage temporaire,
- Solution de data management OMERO.
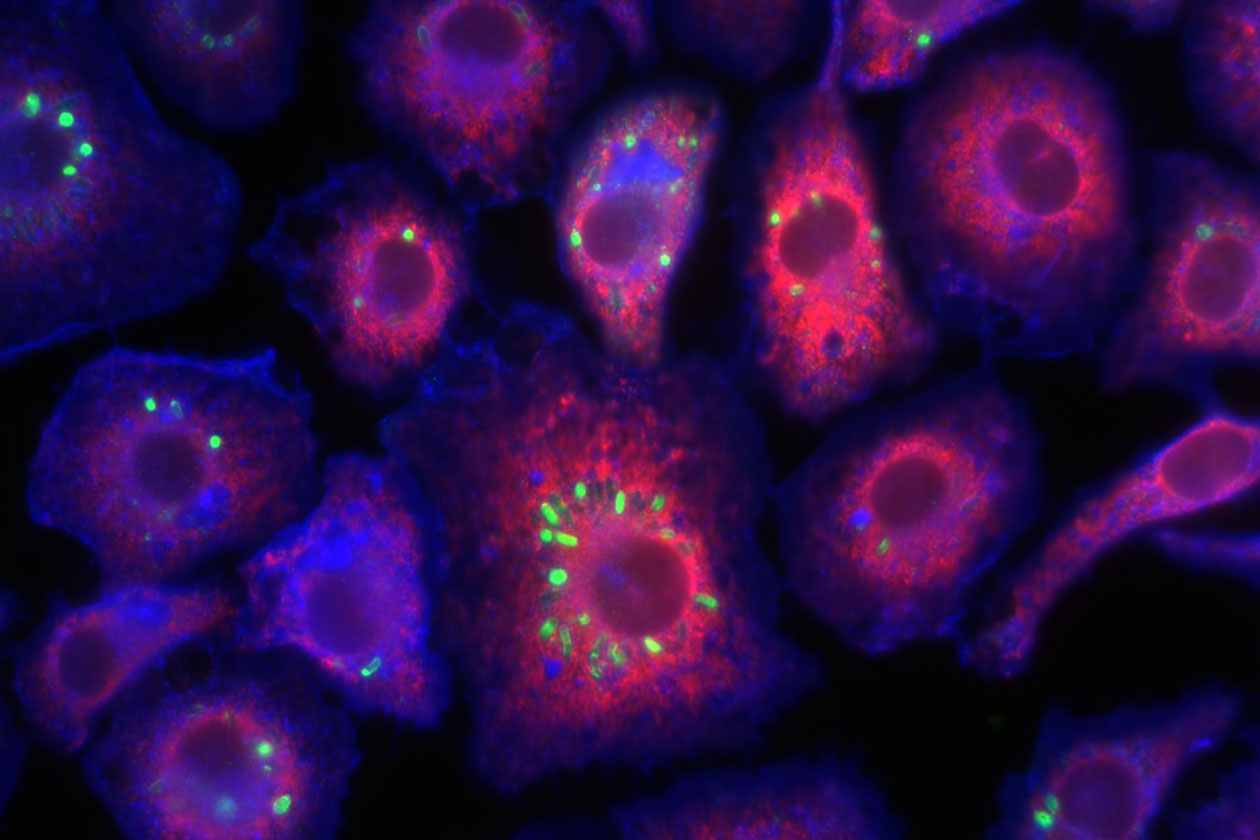
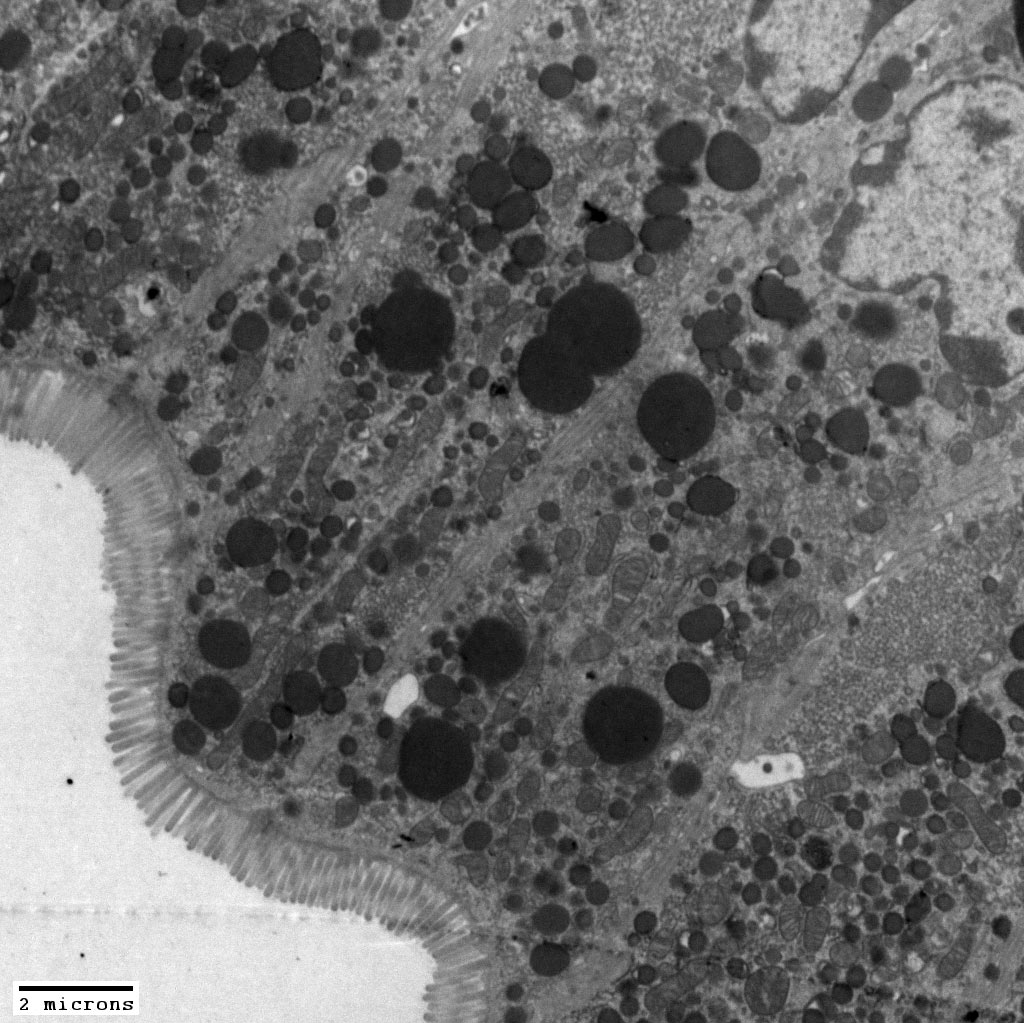
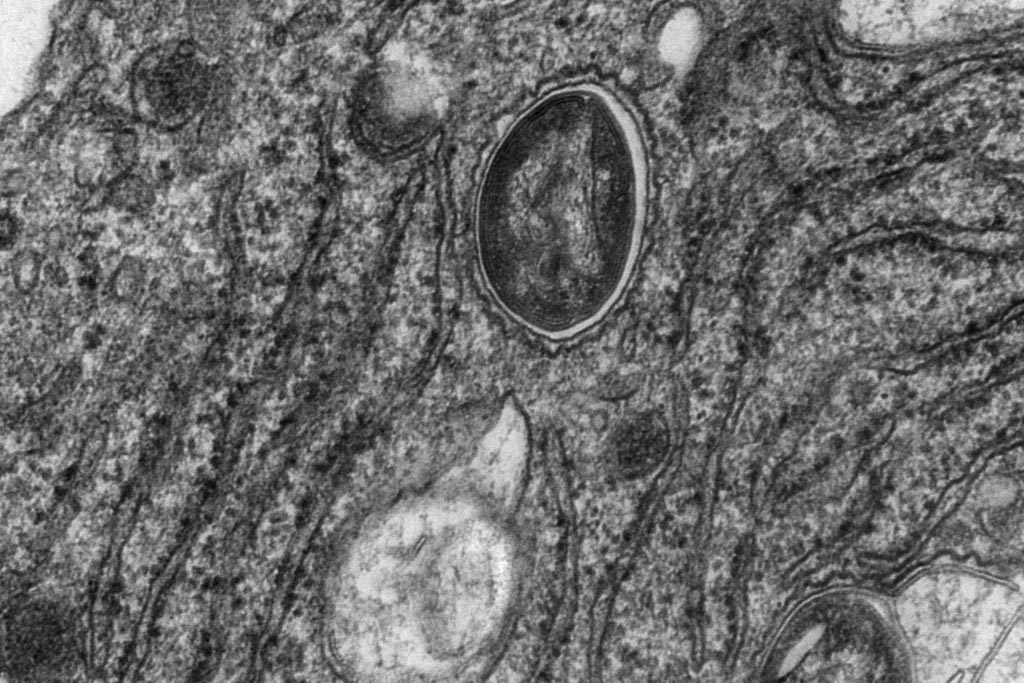



Comment soumettre un projet ?
Le site internet du BICeL présente l’ensemble des domaines, expertises et plateaux de la plateforme. Pour toute demande d’information, de prestation ou de collaboration, vous pouvez envoyer un email à l’adresse bicel-contact@univ-lille.fr. Le responsable du plateau concerné vous recontactera pour identifier avec vous les approches à mettre en œuvre afin de répondre à votre question scientifique, pour évaluer les coûts et formaliser la demande.
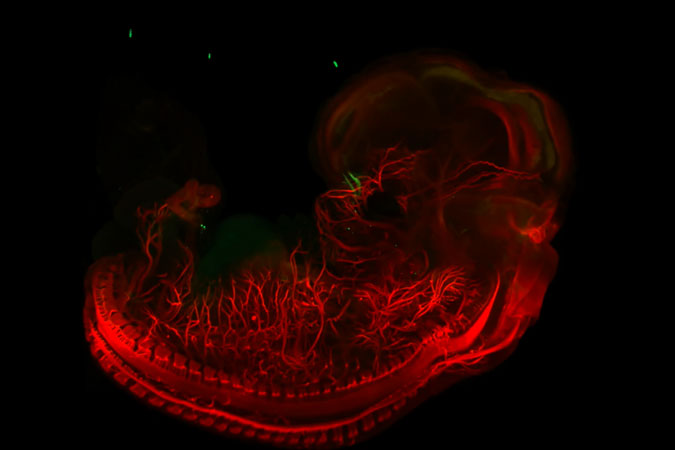
Exemple d'utilisation
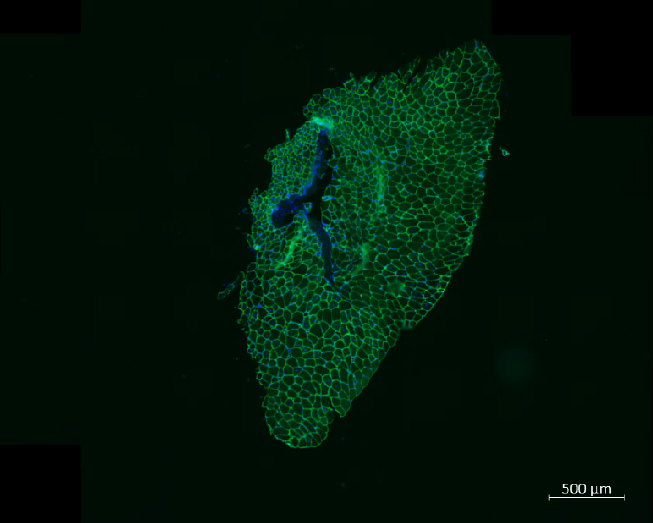
Imagerie 3D de la vascularisation de l'épididyme chez la souris
Longtemps perçu comme un simple tube accessoire du système reproducteur masculin, l’épididyme est désormais reconnu comme un élément clé de la fertilité masculine. Il joue non seulement un rôle crucial dans la maturation et la survie des spermatozoïdes, mais participe également à un rôle immunitaire complexe, en régulant la tolérance aux antigènes des spermatozoïdes, tout en assurant leur protection contre les agents pathogènes. Bien que des progrès aient été réalisés dans la compréhension de son immunobiologie au niveau moléculaire et cellulaire, l’organisation de ses réseaux sanguins et lymphatiques reste mal comprise.
Une collaboration entre le BICeL et des chercheurs de l’Université de Lille et de l’Université de Clermont-Ferrand, a permis de visualiser en 3D la vascularisation de l’épididyme chez des souris adultes matures, ainsi qu’au cours du développement postnatal. Pour ce faire, un modèle de souris transgénique VEGFR3:YFP a été utilisé, en combinaison avec des méthodes d’imagerie 3D à haute résolution, de transparisation d’organes et la détection multiplexe de marqueurs lymphatiques (LYVE1, PDPN, PROX1) et sanguins (PLVAP/Meca32).
Pour en savoir plus : Damon-Soubeyrand. et al. (2023). Three-dimensional imaging of vascular development in the mouse epididymis. eLlife, 12:e82748.
Contact
Bioimaging center Lille (BICeL)
Bâtiment plateformes-cancer
1 place de Verdun
59019 Lille
Région : Hauts-de-France +33 (0)3 20 87 10 40
+33 (0)3 20 87 10 40
bicel-contact@univ-lille.fr
Site de la plateforme
THÉMATIQUES : Histologie, anatomopathologie, Imagerie cellulaire, Imagerie in vivo, radiobiologie
RESPONSABLES SCIENTIFIQUES :
Frank Lafont
RESPONSABLES TECHNIQUES :
Sophie Salomé-Desnoulez
TUTELLES : CHU de Lille, CNRS, Inserm, Institut Pasteur, Université de Lille
LABELLISATION IBiSA : 2010
MOTS CLÉS : Microscopie photonique, Histologie, Microscopie électronique, Cytométrie en flux, Cytométrie spectrale, Imagerie en flux, Microscopie corrélative, Imagerie tissulaire, Imagerie cellulaire, Imagerie corps entier, Imagerie infectieuse, Imagerie intravitale, STED, Multiphoton, Confocal, Feuille de lumière, Spinning disk, MEB, MET, Cancer, Infection, Immunité, Inflammation, Maladies métaboliques, Maladies neurodégénératives
Fiche mise à jour en 2025










